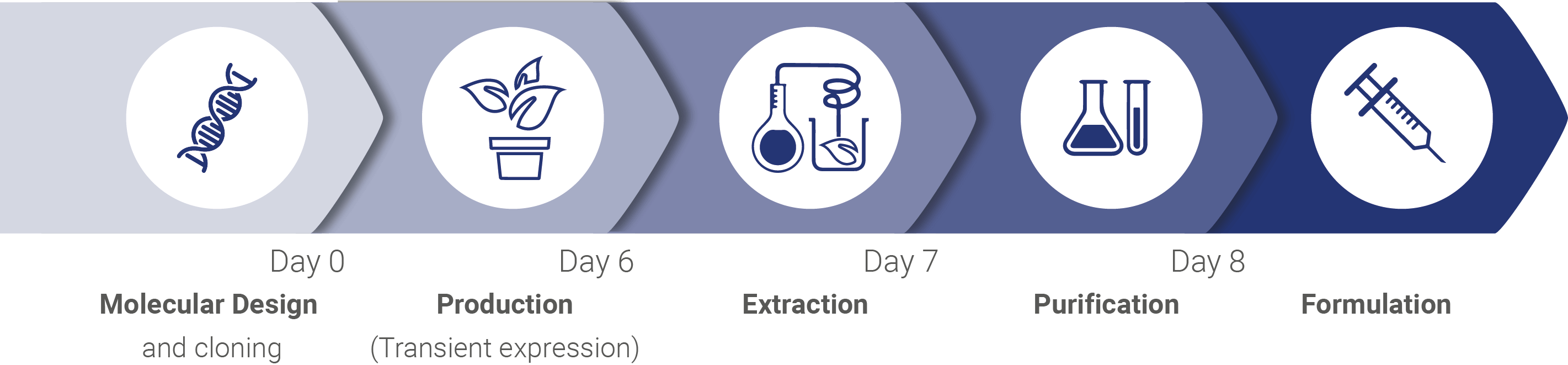
ANGANY is introducing a powerful new approach to the treatment of allergy. The goal: having you claim back a life that can’t wait for allergy. Our technology is the result of over 30 years of R&D in biotechnology, immunology and vaccinology. ANGANY’s promise is based on three major proprietary technological advances.
Our technology
ANGANY wants you to be able to turn the table on allergy
ANGANY’s promise is based on three proprietary technological advances.
First, ANGANY has developed a unique, 3rd generation plant-based production platform. This plant transfection-based production system allows for the efficient production of high-quality (“natural-like”) allergens and other sophisticated proteins for pharmaceutical purposes. ANGANY’s manufacturing system has a short production cycle, is responsive, scalable, economical and GMP compliant. It is also safe and eco-friendly. ANGANY is not a pharmaceutical company centered on one or a few medicines. Ours is what is called an “enabling” technology, one providing for a wide range of potential applications. Although ANGANY’s focus is on allergy, our approach could eventually help people in domains as varied as cancer, autoimmune, endocrinologic, infectious and orphan diseases.
Second, the allergen components (proteins) produced by ANGANY are pure allergens, in their natural form (“natural-like”). Manufacturing such high quality, individually formulated allergens is something the industry has not been able to provide to allergists until now. These allergens, in a soluble formulation, will constitute the key elements of skin tests to come. These will be used in the clinic at the point of care and will allow allergists to accurately identify the allergic dynamics of a patient. No two cases of allergy are the same. Providing each patient with an accurate picture of the allergenic components involved in their condition is a necessary and crucial first step in personalizing optimal treatment for their allergic condition.
Third, ANGANY’s scientists have developed a new approach to the treatment of allergy. Allergic people experience an uncontrolled hypersensitivity reaction when exposed to certain components of their environment. These can be proteins from pollens, dust mites or animal dander. Their immune system becomes sensitized, programmed and ready to respond to any contact with these allergens with harmful consequences. While these common natural components are allergens for allergic patients, the same components do not elicit any “reaction” in non-allergic persons. Their immune system silently eliminates these foreign substances without producing symptoms. Why the immune system of allergic people reacts this way to common compounds is still unknown.
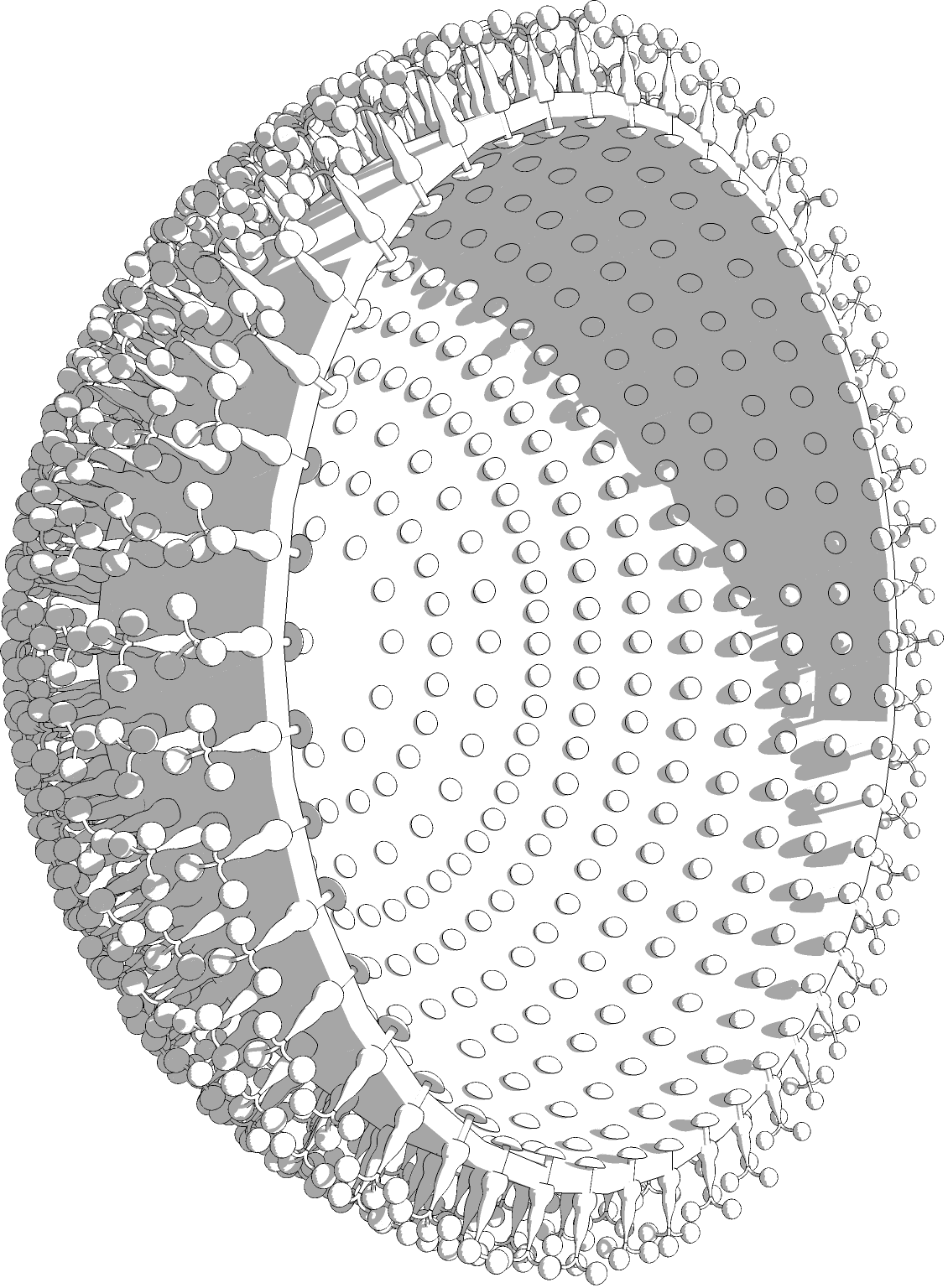
What we do know, is that presenting an allergic immune system to the same allergen, but under a different structure and through a different route, can alter their programmed immune response pattern and establish protection. ANGANY has been able to design and produce allergen bioparticles that present allergens under a 3D structure that triggers a protective immune response rather than a hypersensitive one. ANGANY’s bioparticles are synthesized through its plant-based production system. These bioparticles contain specific membrane lipids that confer auto-adjuvant qualities. We are working hard so that sometime soon allergists can use these particles as true vaccines that will overcome a person’s specific allergy and give rise to prolonged protection.
At this point, ANGANY has produced and purified over 20 allergens and 15 therapeutic auto-adjuvanted allergen-bearing bioparticles. They are currently under pre-clinical testing and in ex-vivo human models.
Sound scientific insights and experienced biopharmaceutical engineering leveraged to give allergy sufferers their life back.
What we have documented so far :
ANGANY’s recombinant allergen products are biogically active and of “natural-like” quality and incomparable purity. They induce an asthma-like allergic response in mice, one identical to that induced by corresponding allergen extracts.
But even more striking :
ANGANY’s allergen bioparticles induce an IgG2a (“normal”) immune response 1,000 times greater than that of standard allergenic extracts typically used in today’s desensitization treatments; more sophisticated ex-vivo human cell culture-based assays have yielded similarly encouraging results. The eBioparticle™ bearing allergens do not cause an allergic response in mice and do not cause degranulation in the blood of dust mite allergic patients. This suggests that this powerful treatment, unlike current desensitization treatments, would not cause a risk of allergic reaction.
Follow us on this journey of discovery
ANGANY has already reached some important milestones. The production of allergens of unique degree of quality and formulations of high purity has been achieved. ANGANY’s bioparticle is uniquely potent and safe as so far determined in separate assays. Its versatility is such that uses in several fields of medicine beyond allergy are now envisioned. Yet, ANGANY cannot rest on its laurels. You and your family are counting on ANGANY’s innovations to help us all win over allergy. Please keep in touch as ANGANY prepares to enter clinical validation in the coming weeks gradually bringing a new, better reality for all suffering from allergy.

